


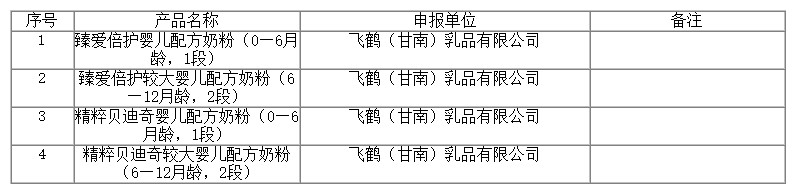
On September 14, 2022, the Center of Food evaluation of the State Administration for Market Regulation updated the list of approval document (decision) for the registration of infant formula product, which includes 4 kinds of infant/older infant formula products, mailing details as below: Zhen’ai Beihu infant formula (0-6 months, stage 1), Zhen’ai Beihu older infant formula (6-12 months, stage 2), Jingcui Beidiqi infant formula (0-6 months, stage 1), Jingcui Beidiqi older infant formula (6-12 months, stage 2),etc.
Need help or have a question?
Send mail